Coronary atherosclerosis, as the main pathological basis of ischemic heart disease, is still managed by statins, antiplatelet therapy and hemodialysis as the main interventions. Notably, nutritional intervention strategies targeting metabolic regulation have shown unique therapeutic potential in recent years, and intermittent fasting (IF) has received widespread attention due to its multiple biological effects.
A recent study published in Life Metabolism by a team of academician Ge Junbo from Zhongshan Hospital, Fudan University, revealed for the first time (Figure 1) that IF-induced production of the gut flora metabolite indole-3-propionic acid (IPA) can mediate attenuation of platelet reactivity through the platelet pregnane X receptor (PXR)-associated signaling pathway, thereby decreasing the platelet activation of patients with coronary artery atherosclerosis and the thrombosis risk in patients with coronary atherosclerosis. This finding provides a new molecular perspective to understand the cardioprotective mechanism of IF.

Antithrombotic drugs encounter “bottlenecks”, whether the IF diet can open up new paths
The strong association between platelet hyperactivity state, a key thrombus-initiating factor in the pathological process of acute coronary syndrome (ACS) and ischemic stroke, and adverse clinical outcomes has been supported by multidimensional evidence-based medicine evidence. Although the dual antiplatelet therapy (DAPT) regimen recommended by current guidelines significantly reduces the thrombotic load in patients with atherosclerotic cardiovascular disease (ASCVD), subgroup analyses based on platelet function assays have shown drug resistance in up to 30-45% of individuals, resulting in a persistent residual risk of ischemic events.
IF, as a dietary intervention strategy to remodel the body’s metabolic phenotype by modulating the eating rhythm, has demonstrated multi-targeted cardiovascular protective effects such as improvement of vascular endothelial function, modulation of lipid metabolic profiles, and inhibition of oxidative stress in preclinical studies. However, there is still a lack of systematic research evidence regarding the direct regulatory mechanism of IF on the platelet activation-aggregation axis and the molecular basis of its synergistic effect with antiplatelet drugs.
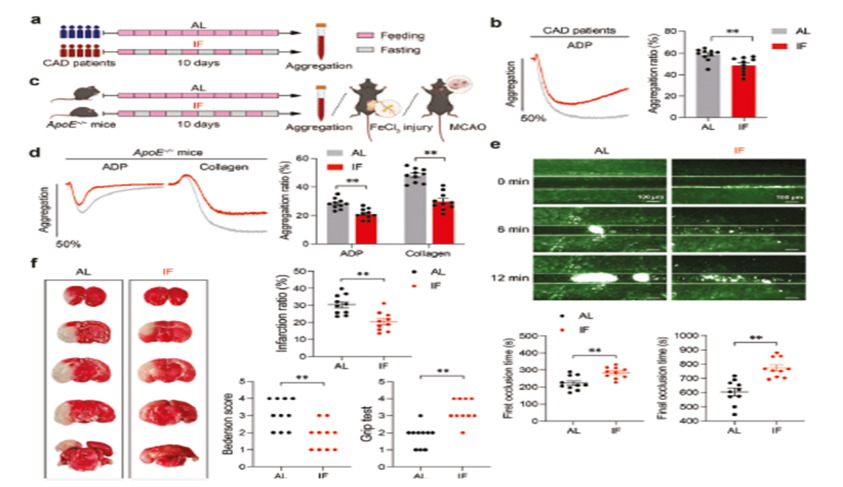
- In this study, we first compared the changes in platelet aggregation rate in patients with coronary artery disease (CAD) and ApoE-/- mice under IF versus ad libitum feeding (AL) conditions by in vitro experiments.
- Subsequently, in vivo experiments were carried out in ApoE-/- mouse models to assess the effects of IF on platelet activation, thrombosis, and cerebral ischemic injury due to middle cerebral artery occlusion (MCAO).
- The key plasma metabolites regulated by IF were further screened by metabolomics.
- Finally, we will focus on the molecular mechanism of IPA, and deeply analyze its interaction with PXR receptor, the regulation of related signaling pathways, and the IPA generation pathway mediated by intestinal flora, so as to comprehensively reveal the cardiovascular protective effect network of IF.
Multifaceted inhibition of platelet activation and thrombosis
1. In vivo mitigation of platelet activation and arterial thrombosis
The results showed that IF significantly inhibited platelet ADP and collagen-induced aggregation in CAD patients. Similar phenomena were observed in the ApoE knockout mouse model. It was further found by the middle cerebral artery occlusion assay that mice in the IF intervention group had significantly reduced the extent of cerebral infarction, significantly improved neurological function scores, and performed better in the motor coordination test. These data suggest that IF may attenuate thrombosis-related lesions by modulating platelet activity (Figure 2).
2. IPA inhibits platelet activation in vitro and attenuates thrombosis in vivo
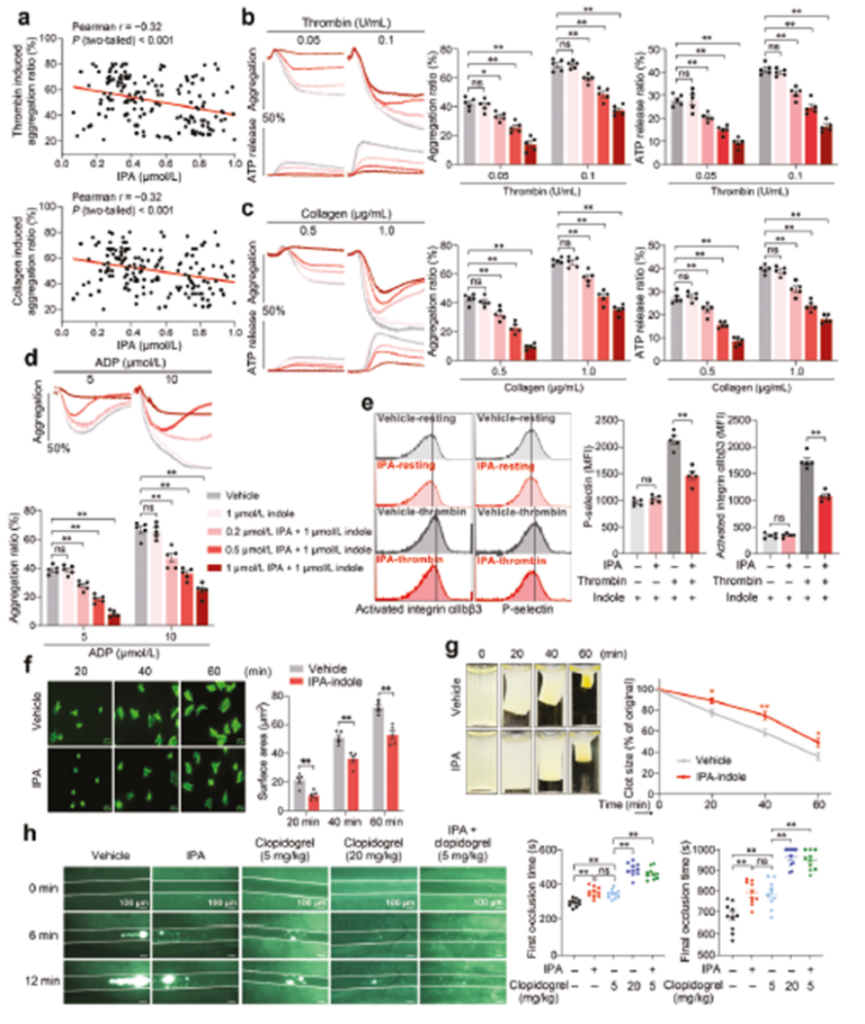
Metabolomics analysis revealed significant effects of IF on serum metabolites in mice. Comparison of the serum metabolic profiles of mice in the IF group and the AL group using LC-MS technology revealed that the levels of orotate and IPA were significantly elevated in the IF group. Further functional validation showed that IPA, but not whey acid, had the biological activity of inhibiting platelet activation, which may be the key mechanism by which IF exerts its antithrombotic effect.
Clinical studies found a significant negative correlation between plasma IPA levels and the degree of platelet aggregation in 160 patients with coronary artery disease who did not receive antiplatelet therapy. In vitro experiments showed that IPA dose-dependently inhibited platelet aggregation and ATP release induced by a variety of agonists (ADP, thrombin, collagen) in the physiologic concentration range (0.2-1 μM). Animal experiments further confirmed that intravenous administration of IPA significantly delayed the process of FeCl3-induced thrombosis in mouse mesenteric arteries, as evidenced by a significant prolongation of both the time to thrombus emergence and the time to complete occlusion (Figure 3).
3. Clostridium perfringens recolonization inhibits platelet activation and thrombosis
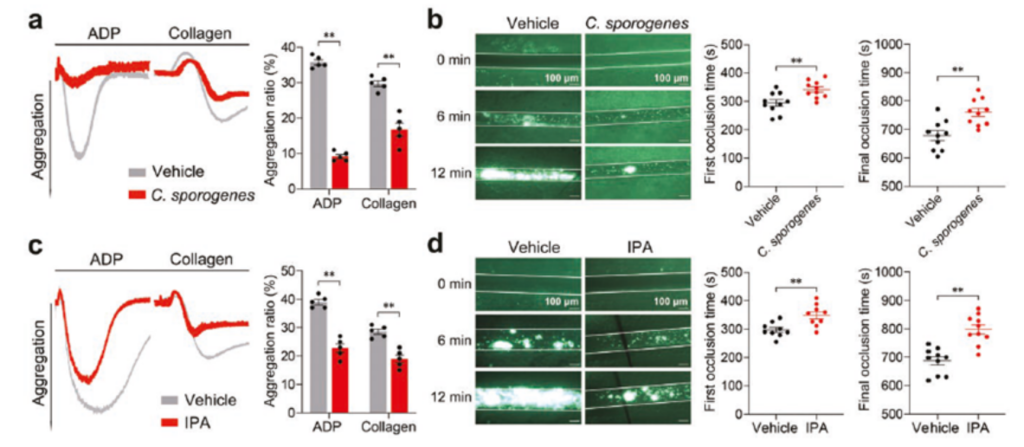
Based on the well-established antiplatelet activity of the intestinal metabolite IPA, the effect of altered intestinal microbiota on platelet activation and thrombosis was next examined. Physiologic IPA is produced primarily by the mouse intestinal Gram-positive bacterium Clostridium perfringens. Therefore, Clostridium perfringens or a carrier was given by oral gavage and platelet aggregation and thrombosis were measured. Platelet aggregation was significantly lower and the time to thrombosis was significantly prolonged in mice administered Clostridium perfringens as well as in mice given direct oral IPA compared to the carrier group. It was shown that Clostridium perfringens could inhibit platelet activation and thrombosis through the IPA metabolic pathway (Figure 4).
Intermittent Fasting’s Unexpected Gain: IF Reduces Myocardial I/R Injury
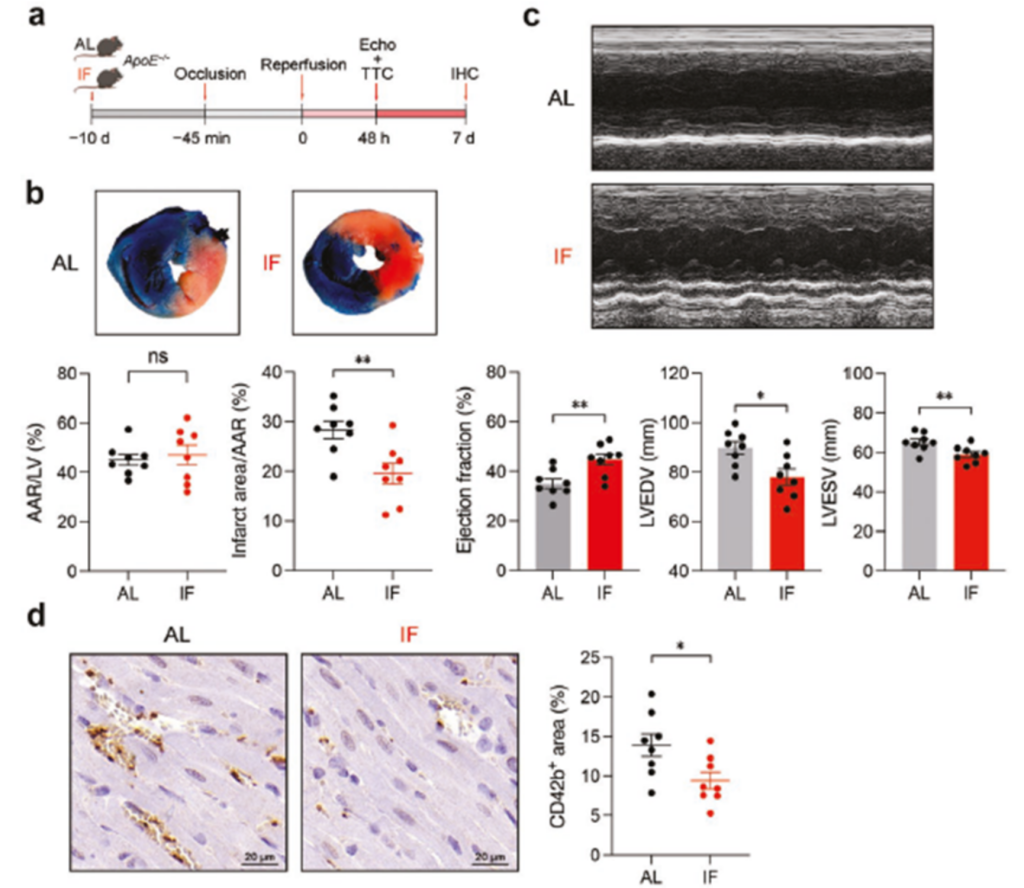
To evaluate the protective effect of IF on myocardial ischemia/reperfusion (I/R) injury: 10 days of IF intervention was implemented in ApoE-/- mice, an acute myocardial infarction model was constructed by ligating the left anterior descending branch (LAD) for 45 minutes, and the infarct area was quantified by double staining method of TTC/Evans blue after 48 hours of reperfusion with the ratio of infarct area and Area at Risk (AAR) ratio and synchronized with cardiac function detection via echocardiography.
The results showed that IF significantly reduced the infarct area/area at risk (AAR) ratio compared with AL diet mice. In addition, IF improved cardiac function, including ejection fraction (EF) and left ventricular (LV) volume index, in mice. Immunohistochemical analysis showed that IF reduced myocardial I/R-induced microthrombi in mouse hearts.
These results suggest that IF may exert cardioprotective effects through multiple mechanisms such as reducing myocardial infarct size, improving cardiac function and inhibiting microthrombosis, providing a new theoretical basis for dietary intervention in patients with coronary heart disease.
In summary, the present study elucidates for the first time a novel mechanism by which intermittent fasting exerts cardioprotective effects via the“gut flora-metabolite-platelet”axis.IF promotes the production of indole-3-propionic acid by intestinal commensal bacteria (e.g., Clostridium perfringens), which inhibits the activation of key signaling pathways, such as Src/Lyn/Syk and LAT/PLCγ/PKC/Ca²⁺, by binding specifically to the platelet PXR receptors and ultimately regulating platelet function. Lyn/Syk and LAT/PLCγ/PKC/Ca²⁺activation of key signaling pathways, ultimately achieving platelet function regulation.
This finding not only expands our understanding of diet-flora-host interactions, but also provides new ideas for cardiovascular disease prevention and treatment, which may lead to the development of novel antiplatelet therapies by targeting and regulating intestinal flora metabolites or PXR signaling pathways. Future studies can further explore the optimal time window for IF intervention, individualized implementation protocols, and the potential for drug development of IPA analogs, providing theoretical support for precision nutritional intervention in cardiovascular disease.



