The intervertebral disc consists of a central nucleus pulposus tissue, a peripheral annulus fibrosus, and a cartilaginous endplate above and below, a unique structure that isolates the nucleus pulposus from the host’s immune system. Certain substances expressed in the intervertebral disc have an inhibitory effect on immune cell and cytokine infiltration, and thus the disc is considered an immune amnesty tissue.
However, during degeneration of the intervertebral disc, its physical barrier is damaged, and the nucleus pulposus may be recognized as a foreign antigen by the immune system; at the same time, inflammatory cytokines in the degenerated disc recruit a large number of immune cells, which disrupts the original immune balance of the nucleus pulposus, leading to the progression of intervertebral disc degeneration.
Recent studies have confirmed that immune cells such as macrophages and mast cells infiltrate the degenerated discs, and that the phenotypic characteristics and number of immune cells are closely related to the degenerative process of intervertebral discs. In terms of therapeutic strategies, mesenchymal stem cell therapy, gene therapy, growth factors and other biologics for the regulation of the immune microenvironment of the degenerated intervertebral discs have entered the stage of animal experiments, and small molecule drugs have also demonstrated a unique potential for regulating the immune amnesty state of the intervertebral discs.
Intervertebral disc degeneration is the main cause of chronic low back pain, and the degenerative spinal lesions caused by it not only seriously affect the quality of life of patients, but also bring a huge economic burden to individuals and society.In recent years, with the rapid development of biotechnology and medical engineering, new therapeutic modalities such as stem cell therapy, gene therapy, and biomaterials and tissue engineering have achieved remarkable results in the treatment of intervertebral disc degeneration. However, the exact pathogenesis of disc degeneration, especially the role of disc immune imbalance in the disease process, remains unknown.
Immune amnesty is a property of certain specific sites during the development of an organism, which are able to avoid direct contact with immune cells and prevent recognition and attack by the immune system even in the presence of antigenic substances, thus effectively avoiding tissue damage and dysfunction due to immune reactions. This property is essential for maintaining the stability and function of the tissues involved.
Tissues with immune amnesty, such as heart valves, eyes, brain, testes and embryos, have been studied in depth. The mechanism of isolation of these tissues from the immune system in the host has received extensive attention and has been thoroughly explored. The intervertebral disc, as the largest avascular tissue in the human body, consists of a central nucleus pulposus, a surrounding fibrous annulus, and a cartilaginous endplate above and below. This unique anatomical structure isolates the nucleus pulposus from the host’s immune system, giving the disc the characteristic of an “immune amnesty organ”.
However, during disc degeneration, degenerating nucleus pulposus cells can release a large amount of inflammatory mediators, which can recruit immune cells to migrate into the disc, thereby triggering or exacerbating localized inflammatory responses and accelerating the process of disc degeneration. Therefore, the purpose of this paper is to systematically review and explore the causes and mechanisms of the disc immune amnesty phenomenon, as well as to analyze the various types of immune cells involved in disc degeneration and the clinical significance of the immune response to disc degeneration, with a view to providing new theoretical foundations and technical support for the prevention and treatment of intervertebral disc degeneration.
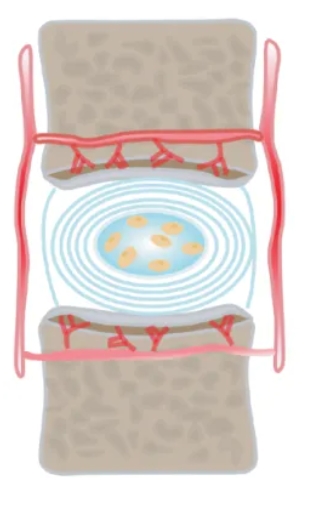
Causes and mechanisms of intervertebral disc immune amnesty
(i) Physical immune barriers
As we age, the number of blood vessels in the deeper layers of the intervertebral disc decreases and their caliber becomes smaller accordingly. This change may be related to the high concentration of proteoglycans in the nucleus pulposus and the inhibitory effect of the high pressure environment on blood vessel growth . As the largest avascular structure in the human body, the absence of blood vessels in the nucleus pulposus is a major obstacle to immune cell infiltration, which means that immune cells cannot reach the nucleus pulposus directly through the blood pathway. The avascular nature of the nucleus pulposus and the physical barrier provided by the annulus fibrosus and cartilage endplates together form the basis of the intervertebral disc as an immune-immune amnesty tissue. This unique structure not only ensures that the nucleus pulposus is protected from the direct influence of the immune system, but also provides an important safeguard for the disc to maintain normal physiological function.
(ii) Molecular immune barrier
Immunosuppressive molecules expressed in the intervertebral disc exhibit significant inhibitory effects on immune cells entering the region, helping to remove small numbers of invading immune cells and thus maintaining the immune amnesty state of the disc.Fas ligand (FasL), a potent inducer of apoptosis, is widely distributed in a number of known immune amnesty regions [ 15 , 16 , 17 , 18 ]. regions.
Previous studies have demonstrated that normal intervertebral disc nucleus pulposus cells are also positive for FasL, which not only induces apoptosis of key immune cells, such as macrophages and CD8+ T cells, to remove infiltrating immune cells, but also triggers apoptosis of vascular endothelial cells to maintain the avascular nature of the nucleus pulposus tissue.
Thus, FasL may play a critical molecular barrier function through its dual mechanism of vascular endothelial cell-mediated apoptosis and immune cell apoptosis, protecting the intervertebral disc from immune responses while maintaining its unique physiological environment. This finding provides a new perspective for understanding the immunoregulatory mechanism of intervertebral discs and opens up potential pathways for exploring therapeutic strategies for related diseases.



